Sublimation is when a solid becomes a gas without turning into a liquid first.
There are three states of matter. Adding or removing energy from a substance can change the state it is in. For example water is a solid below 0oC ( freezing point ), a liquid above 0oC ( melting point ) and a gas above 100oC ( boiling point ).
Boiling, melting and freezing point temperatures are different for different substances.
What is an example of sublimation?
One example of sublimation is when dry ice ( solid carbon dioxide ) turns from ice to carbon dioxide gas when it comes into contact with air.

The opposite of sublimation is deposition.
What is an example of deposition?
An example of deposition is when frost forms from water vapour on cold ground or a window pane on a cold day.
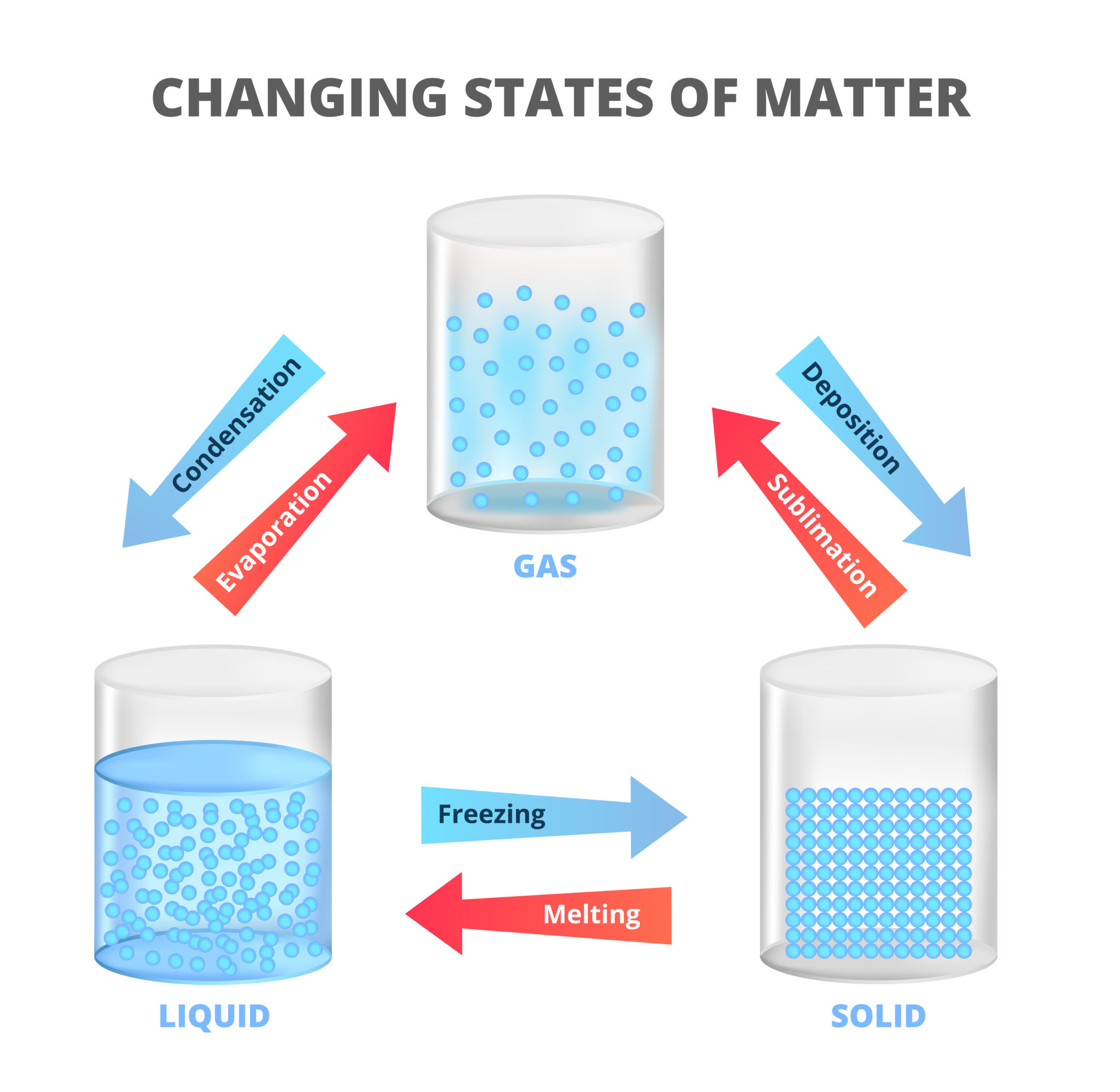
These changes are known as physical changes as they can be reversed by heating or cooling. The final substance is the same matter as before the physical change. The opposite of a physical change is a chemical change which cannot easily be reversed. The final substance is not made of the same matter as before the change.
The post What is sublimation? appeared first on Science Experiments for Kids.
